The CBD-based therapy, developed by the biotech pharma company ANANDA Scientific, has been cleared for a clinical trial
By Jason Langendorf
People with opioid use disorder (OUD) may soon have a non-opioid-based medication as an option for treatment. But more research—and likely more myth-busting—will be required before the proposed therapy comes to pass.
Earlier this month, the biotech pharma company ANANDA Scientific announced a clinical trial for evaluating a cannabidiol (CBD)-based drug treatment called Nantheia ATL5. The Food and Drug Administration (FDA) has already approved an Investigational New Drug (IND) application for the trial, which will be conducted at UCLA’s Icahn School of Medicine at Mount Sinai and funded by the National Institute on Drug Abuse (NIDA).
“This clinical study at UCLA is an important component of our clinical development efforts focused on opioid addiction, where a non-addictive therapy is a significant unmet need.”
—Sohail R. Zaidi, CEO, ANANDA Scientific
“This is the fourth IND approval for our investigational drug Nantheia product line, and it further reinforces our vision of developing CBD as a therapeutic for a number of key indications,” said Sohail R. Zaidi, ANANDA’s chief executive officer. “This clinical study at UCLA is an important component of our clinical development efforts focused on opioid addiction, where a non-addictive therapy is a significant unmet need.”
Why This Treatment Matters
Coming off a year in which, for the first time, overdose deaths in the U.S. topped 100,000 over a 12-month period—with opioids accounting for 75% of those fatalities—addiction experts generally agree that no stone should be left unturned in the search for effective therapies in the treatment of OUD. Currently, however, the FDA has approved only three medication-assisted treatments (MATs) for treating OUD:
- Buprenorphine
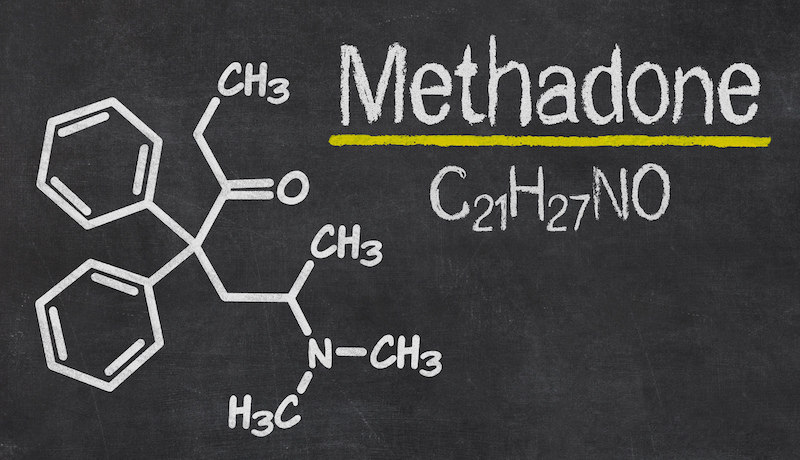
- Methadone
- Naltrexone
Although each has been deemed safe and effective when used as directed and in combination with behavioral therapy, each—like many medications—comes with potential side effects and some risk of diversion. On its website, the FDA notes that the treatment of OUD “means helping more people secure MAT, which requires us to break the stigma often associated with some of the medications used to treat OUD. It also requires us to find new and more effective ways to advance the use of medical therapy for the treatment of OUD.”
If a CBD-based MAT therapy can be developed, patients with OUD would have a non-opioid-based treatment medication alternative for the first time, and many of the (mostly unfounded) fears of opioid MAT critics would become moot.
Although cannabidiol has been the subject of certain wildly false claims from rogue manufacturers—it does not cure cancer or COVID—it has shown promise as what Harvard Medical School describes as “a helpful, relatively non-toxic option for managing anxiety, insomnia and chronic pain.” If a CBD-based MAT therapy can be developed, patients with OUD would have a non-opioid-based treatment medication alternative for the first time, and many of the (mostly unfounded) fears of opioid MAT critics would become moot.
But Isn’t CBD Actually Weed?

Perhaps most significant beyond its potential effectiveness is that CBD is not a form of cannabis. Again, from Harvard Medical School: “While CBD is an essential component of medical marijuana, it is derived directly from the hemp plant, a cousin of marijuana, or manufactured in a laboratory.” According to the World Health Organization, “CBD exhibits no effects indicative of any abuse or dependence potential. … To date, there is no evidence of public health-related problems associated with the use of pure CBD.”
Nantheia ATL5, an oral therapy containing 100mg of CBD per capsule, features proprietary delivery technology (Liquid Structure) touted as enhancing the effectiveness and stability of CBD. A cannabidiol-based MAT option should allay the usual “trading one drug for another” criticisms. At the end of the day, however, clinical trials will need to prove it safe and effective—up to the same FDA standards as buprenorphine, methadone and naltrexone.
Photo: Shutterstock