The CEO of Ensysce Biosciences is spearheading the development of painkillers that are designed to eliminate the risk of prescription opioid misuse and addiction
By Jenny Diedrich
Lynn Kirkpatrick, PhD, one of the few female CEOs in the pharmaceutical industry, is leading opioid pharmacology into the next generation using what she describes as “clever chemistry.”
Kirkpatrick is CEO of Ensysce Biosciences, a biotech company based in La Jolla, Calif., that specializes in advanced and novel opioid pharmaceutical science. The company’s products, which are in the approval phase and not yet available to patients, are designed to provide virtually the same level of pain relief as current prescription opioids without the risk of misuse and addiction. In a nod to the company’s forward-thinking approach, its work is called the Opioid 4.0 Project.
Under Kirkpatrick’s leadership, Ensysce has been awarded Fast Track designation by the Food and Drug Administration (FDA) in bringing its medicines to market. They are currently in the clinical stages, fueled by more than $60 million in funding.
“I started with designing drugs, and it’s wonderful to feel like you’re actually taking something across the goal line and helping patients on the other side,” Kirkpatrick says.
“We have to understand how better to use some of the best pain-relieving medications properly and responsibly. … We’re really trying to develop something that’s better for the patient, gives prescribers some comfort and eases the anxiety of taking pain medication.”
—Lynn Kirkpatrick, CEO, Ensysce
TreatmentMagazine.com recently talked with Kirkpatrick about her company’s clever chemistry and how its products could help curb the opioid epidemic.
Q. Tell us about your background in opioid science.
A. My background is in medicinal chemistry. I started my career designing drugs, with the focus initially in the oncology space. I spent 16 years in academia teaching pharmacology and medicinal chemistry. I started my first company in the 1990s, and subsequently Ensysce acquired this technology [that the company is utilizing], which has fascinating chemistry associated with it.
In academia, you can do the basic research, but it doesn’t allow one to translate that and provide a product to the end user—the patients. I thought [starting a company] would give me the opportunity to move from discovering something to developing it and really providing something novel for the end user. I’ve pivoted from oncology into the pain focus over the last 10 years or so. With this incredible chemistry, I’m able to offer something in the pain and substance abuse space.
Q. What products does Ensysce Biosciences have under development?
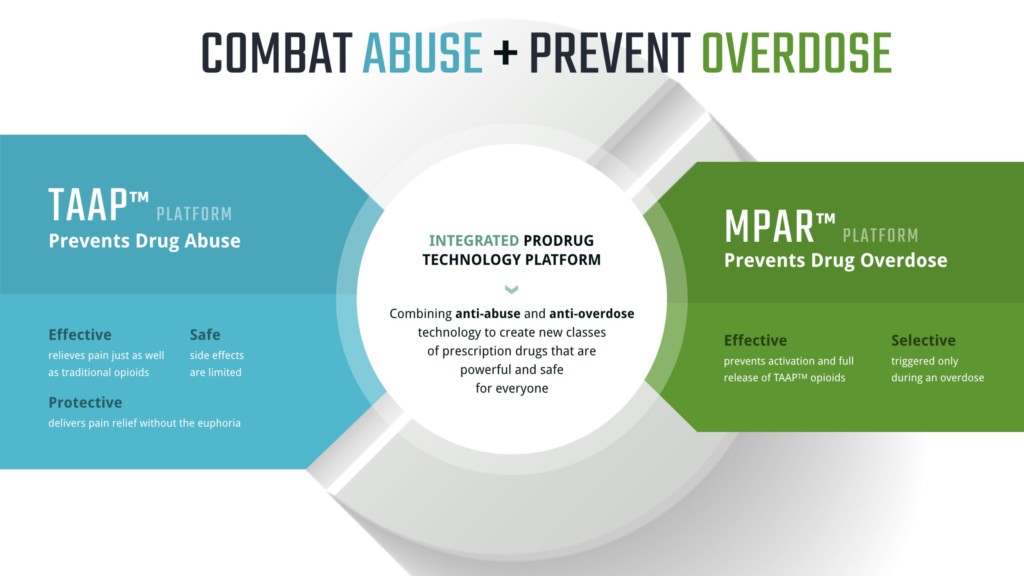
A. We’re using clever chemistry to solve difficult problems associated with a lot of prescription drugs. The focus of our lead program is in the opioid space. We have two technology platforms. The initial platform uses this clever chemistry to modify drugs and make them inactive unless they’re swallowed properly. It removes the ability to abuse them for recreational uses or by accident.
The first platform, called Trypsin Activated Abuse Protection (TAAP), refers to the enzyme the body uses to activate and make the products work. Trypsin is an enzyme that everyone has. It’s used to digest meat products, and it’s secreted and activated in the small intestine. The reason our chemistry is so clever is that our products are recognized by this enzyme, and our body actually starts the activation. We only have trypsin in the gut, so that’s why chewing doesn’t change the profile of the drug. You can’t snort it, and you can’t inject it.
“Our TAPP products are like a two-step verification going into your bank account.”
—Lynn Kirkpatrick
We also have combination products, and this is our overdose protection platform. We add a little bit of trypsin inhibitor in with the TAPP product. It sounds counterintuitive—why would you inhibit the drug you need? When you only have a small amount, it really doesn’t affect the activation. If you inadvertently forget whether you’ve taken your medication and take another dose, making you at risk of side effects or overdose, that inhibitor blocks the activation so you don’t get too much of the product.
Q. What is the overall benefit of Ensysce’s products for patients?
A. We’re providing safer products for individuals who may be concerned about taking or having them lying around in their medicine cabinets. Because of the profile of the drugs, it may give people who are hesitant to use painkillers some confidence that they have this potentially safer product. You can still get a powerful pain reliever with some safety factors built in.
The unique aspect with our chemistry is that our TAPP products are like a two-step verification going into your bank account. Trysin starts the activation, but there’s a second step that dictates how quickly the material is released into the body. Chemically, we’ve designed agents to be extended-release, but we also have immediate-release products still with this protection of having to be swallowed, and the chemistry releases it more quickly. We can have products for the acute pain space or more chronic [pain].
Q. Ensysce has called its work the Opioid 4.0 Project. What does that refer to?
A. Opioids have been used for thousands of years. They were discovered around 3400 BC. They were extracted and used as tinctures for a long period of time, and then the pharmaceutical industry was able to isolate the opioids from the product, which we refer to as the second generation. When extended-release formulations came out, we call this category generation three.
“Generally, people using opioids start with prescription drugs. If we can reduce the abuse of prescription opioids, we hope we can start reducing the population of individuals who are in opioid crisis.”
—Lynn Kirkpatrick
We’re introducing our top products as the next generation of opioids, the fourth generation. We believe we’re entirely different. We’re not a formulation; we’re using clever chemistry to give this two-step activation reaction. We can limit where it’s activated and control the timing of release, so we’re calling this Opioid 4.0, or the next generation of opioids.
Q. Is Opioid 4.0 reflective of a new approach by the pharmaceutical industry?

A. I think the attitude is starting to change. Some people are totally against opioids for pain relief. There have been tapering and restrictions, and politicians getting into the mix. I think now people recognize there has been an impact on those who suffer from pain. We have to understand how better to use some of the best pain-relieving medications properly and responsibly. I believe that’s where Ensysce comes in. We’re really trying to develop something that’s better for the patient, gives prescribers some comfort and eases the anxiety of taking pain medication. We’re not only providing a highly unique product, but we’re trying to also build in some of the more social benefits for individuals with pain.
Q. How might Ensysce drugs help curb help the opioid epidemic?
A. Generally, people using opioids start with prescription drugs. If we can reduce the abuse of prescription opioids, we hope we can start reducing the population of individuals who are in opioid crisis. We are not going to solve the problems for those individuals who are currently addicted to opioids. Some abuse comes from wanting that instant gratification. People chew the product, they snort the product, they extract and inject. We’ve demonstrated with ours that’s ineffective in changing how the opioid is released. You still have to swallow ours, and it takes time for the release, so it’s not going to provide instant gratification.
Q. What are your thoughts on the culpability of the pharma industry in sparking the opioid epidemic?
A. The pharma industry is blamed for a lot of things—high prices, marketing. Probably mistakes were made. I think, generally, a lot of the physicians who treat patients with pain who are being blamed, as well as the companies that are being brought into litigation, are really trying to do the right thing. There are bad actors everywhere. I’m not pointing fingers, but really the intention has always been to help people in pain. Whether it was done properly is questionable.
There can be finger-pointing all around—the industry, regulators, everyone involved who has created this. And it’s not only in the U.S. Problems are emerging around the globe, and it’s not necessarily just the pharmaceutical industry. There are social problems with drugs that need to be solved in different ways. We know COVID has contributed drastically to the crisis over the last couple years. There are so many social factors, as well.













