Plus: An unexpected call to legalize cocaine, a potential naloxone implant and more addiction treatment industry news
By Mark Mravic
New & Next: Policy
Pew Lays Out a Set of Standards to Measure Addiction Care
The growing opioid crisis has created consensus in the U.S. around the urgent need to improve addiction treatment, curb overdoses, save lives and support recovery. But the great challenge for health officials, policymakers and providers is that there is no consensus around measuring the quality of treatment. That lack of coherence and consistentcy shouldn’t be a surprise, given how highly fragmented the field is, with public, nonprofit and for-profit providers; treatment settings that include hospitals, community clinics, primary care offices, inpatient facilities, prisons and more; a payment landscape that is hard to understand and difficult to navigate; and a widely diverse patient population.
Basic numbers on opioid use disorder (OUD) are notoriously hard to come by, or to compare from community to community: Most states do not currently track the number of people diagnosed with OUD, the number of providers available, the use of approved medications or treatment retention and outcomes.
“It’s time for state officials to apply [a] data-driven approach to OUD treatment and use these measures to assess and ultimately improve treatment systems. Doing so will save lives.”
—Pew Charitable Trusts
To address that challenge, last year the Pew Charitable Trusts’ Substance Use Prevention and Treatment Initiative convened a panel of stakeholders—including government officials, treatment providers, advocates, data experts and people with lived experience—to develop a standard set of metrics to measure OUD treatment. On Tuesday, Pew released its recommendations. The core comprises eight measures based on the cascade of care model that, if adopted, “would help states understand their treatment systems’ capacity to effectively diagnose, treat, and support patients with OUD.”
The metrics are:
- OUD diagnosis: Percentage of people who had a documented OUD diagnosis (e.g., on an insurance claim)
- Percentage of people assessed using a standardized screening tool: Rate of assessment using a tool such as the Drug Abuse Screening Test (DAST) or the Tobacco, Alcohol, Prescription Medication, and Other Substance Use (TAPS) tool
- Use of pharmacotherapy for OUD: Percentage of people diagnosed with an OUD who received medication to treat it
- OUD provider availability: Number of providers and treatment programs that can provide medication for opioid use disorder (MOUD)
- Continuity of pharmacotherapy for OUD: Percentage of people receiving MOUD who use it for at least six months
- Initiation of OUD treatment and engagement in OUD treatment: Percentage of people who initiate treatment within two weeks of diagnosis; percentage of people with two or more services within the first month after initiating treatment
- Follow-up after an emergency department visit for substance use: Percentage of people who receive follow-up care for substance use disorder or an overdose within seven days, and 30 days after visiting an emergency department for a substance use disorder-related issue
- One or more patient-reported outcome measures to be determined by each state: Percentage of individuals who achieve an improved level of functioning or quality of life
Collecting the data, however, isn’t sufficient. For the measures to work, Pew says, states must put the information to effective use, including:
- Publicly reporting OUD treatment data collected via the proposed measures
- Designing a plan to use the collected data, including regularly reviewing the data and using it to inform policy change
- Involving people with lived experience and providers in data-driven decision-making
- Breaking down data by factors such as race and ethnicity to improve health equity and identify communities most in need of resources
In introducing the recommendations, Pew writes, “Policymakers have long used data to inform their decision-making. It’s time for state officials to apply this same data-driven approach to OUD treatment and use these measures to assess and ultimately improve treatment systems. Doing so will save lives.”
The full report can be found here.
The Economist calls for Cocaine Legalization
The move for drug decriminalization makes for strange bedfellows. Case in point: The Economist, the British-based magazine that is one of the stalwarts of respectable conservative media, has called for the legalization of cocaine. Deeming President Joe Biden’s recent marijuana pardons “too timid,” the op-ed notes that despite decades-long efforts in both the U.S. and Latin America to curb cocaine production and trafficking—including tens of billions of dollars pumped into Colombia alone—global production hit a record high in 2020, with no respite in sight.
The answer, The Economist says, “is full legalisation, allowing non-criminals to supply a strictly regulated, highly taxed product, just as whisky- and cigarette-makers do. (Advertising it should be banned.)” Legal, regulated cocaine, the piece argues, would be less dangerous than the often-adulterated street product; the move would also “defang” drug gangs and reduce violence, especially in Latin America; and it would bring political stability to volatile regions where cocaine production and trafficking have caused endemic corruption. “Just now, full legalisation seems politically impossible: few politicians want to be called ‘soft on drugs,’“ the unsigned opinion piece reads. “But proponents must keep pressing their case. The benefits—safer cocaine, safer streets and greater political stability in the Americas—far outweigh the costs.”
New & Next: Research
Biotech Startup Receives Grant to Develop Naloxone Implant
Rescue Biomedical, a startup from a team of scientists at Purdue University, has received a four-year, $2.82 million grant from the National Institutes of Health’s Small Business Innovation Research (SBIR) program to develop an under-the-skin implant that will detect opioid overdoses and immediately deliver naloxone, the opioid-reversal drug. The grant will allow the team to research client needs and identify a regulatory pathway, then perform usability tests and demonstrate feasibility. Additional funding will be needed at that point to begin clinical trials and scale up manufacturing.
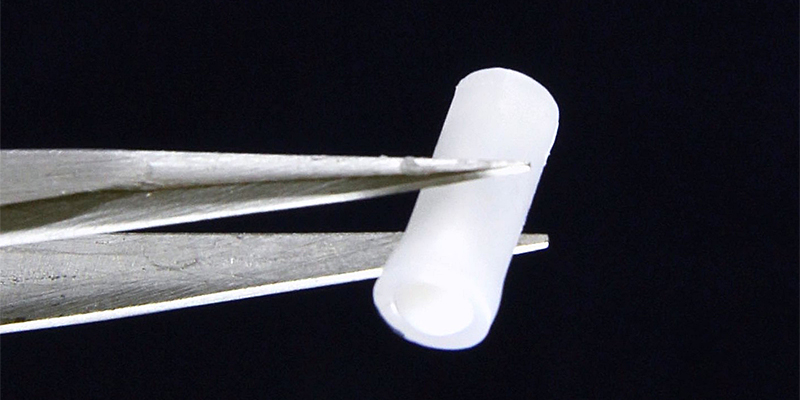
Rescue Biomedical CEO Hyowon “Hugh” Lee, PhD, who heads Purdue’s Center for Implantable Devices, said the company hopes to partner with recovery clinics and clinicians to identify and work with OUD patients at high risk of overdosing again: “Our device is a closed-loop drug delivery system that automatically detects when someone is overdosing and immediately provides life-saving naloxone to prevent long-term neurological damage or death.” He also praised the grant program for allowing the research to get underway: “We are trying to work on solving real-life problems that affect hundreds of thousands of people across the country, and the SBIR program from the NIH is enabling it.”
New & Next: People
NAADAC Welcomes Maxwell as New President
Angela E. Maxwell, PhD, CPS, has officially begun her two-year term as president of NAADAC, the Association of Addiction Counselors. In her role, she will oversee the organization that represents 100,000 addiction counselors, educators and other healthcare professionals in the U.S., Canada and abroad.

Maxwell, the first Black president of NAADAC and the first to be a certified prevention specialist, has 25 years of experience in substance use prevention. “I am committed to bringing to bear and elevating the voices of everyone who is impacted and is struggling and suffering from addiction. That is my commitment to you all,” Maxwell said at NAADAC’s 2022 conference and 50th anniversary celebration in Indianapolis. “I look forward to working with the staff, the executive committee, the board of directors, and all of you over the next two years.”
In addition, NAADAC announced its executive committee for the next two-year term.
New & Next: Facilities
Eleanor Health, an outpatient addiction and mental health treatment provider at the forefront of value-based payment models, has opened its first clinic in Texas. The facility in San Antonio brings the number of Eleanor clinics to 31 across seven states: Louisiana, Massachusetts, New Jersey, North Carolina, Ohio and Texas. In addition to in-person care at the San Antonio facility, virtual care will be provided across the state of Texas.
Value-based care is meant to align the priorities of patients, payers and providers by incentivizing the reduction in total cost of care while improving key quality measures. Eleanor’s approach includes pharmacotherapy, psychiatry, individual and group therapy, peer recovery and nurse care management.
The rise in overdose deaths “is bearing out both nationally and in Texas, underscoring the immediate need for substance use disorder treatment rooted in harm reduction,” said Annette Betancur, MBA, RN, Texas director of operations at Eleanor Health. “I look forward to spearheading operations for Eleanor’s clinical model in San Antonio and across Texas.”
New & Next: Events
Coming This Month: National Takeback Day
Oct. 29, marks the 23rd National Prescription Drug Takeback Day. An initiative of the U.S. Drug Enforcement Agency (DEA), the program raises awareness of the dangers of unneeded or leftover medications and provides resources for people to dispose of their meds. Foremost is a locator that allows users to plug in their zip code or city and find the nearest collection site.
The most recent Takeback Day, in April 2022, saw 4,427 law enforcement agencies in the U.S. participating across 5,144 sites, and 360 tons of medications collected. Since its inception, Takeback Day has safely disposed of nearly 8,000 tons of unused prescriptions.
Top photo: Scott Graham; Implant: Hugh Lee